Patient Education for Spinal Cord Stimulator Placement
The spinal cord stimulator (SCS) procedure aims to alleviate chronic pain using gentle electrical impulses to modify pain signals to the brain. It is used to treat enduring, challenging-to-treat pain in the trunk or legs. Positioned near the middle of the spinal cord in the back (around T8 level), the device emits mild electric pulses that interrupt pain perception. The battery, or generator, requires replacement approximately every 5 years.
Benefits of SCS Placement:
- Reduced pain: SCS can provide relief from chronic pain that has not responded well to other treatments.
- Improved quality of life: Pain reduction can lead to better sleep, increased activity, and an overall improved quality of life.
- Decreased reliance on medications: SCS may allow you to reduce the need for pain medications.
While SCS generally reduces pain, complete pain relief is uncommon. SCS involves a one-week trial period to evaluate effectiveness, followed by permanent implantation if the trial is successful. Patients do not undergo final implantation if they are unsatisfied with the trial results.
Step 1: Trial
A spinal cord stimulator trial is a temporary procedure aimed at assessing the potential effectiveness of an SCS in managing chronic pain before committing to a permanent implant. This trial serves as a crucial step because it helps both the patient and their healthcare provider determine if a long-term implant of the spinal cord stimulator is a suitable option.
During the trial, thin wires with electrodes (leads) are placed near the spinal cord and connected to an external generator. The trial usually lasts a week. Afterward, the wires and generator are removed for assessment. Successful pain relief and improved quality of life during the trial indicate potential benefits for a permanent implant. Unsuccessful pain relief suggests that permanent placement should not be pursued.
Step 2: Permanent Placement
Anesthesia: You will receive either general or local anesthesia for your comfort during the procedure.
Lead placement: The leads are inserted into the epidural space near your spinal cord under X-ray guidance. A thoracic laminectomy (bone removal) is performed through a mid-back incision (typically around T10), creating a window to pass the paddle lead (Figure 1). The paddle lead wire is then tunneled under the skin to a subcutaneous pocket above the belt line, where the battery-generator is positioned (watch video). However, in cases of scarring or narrow canal diameter, the thoracic paddle lead might not be feasible, and wire leads, similar to the trial, are used (watch video).
Generator placement: The generator is usually implanted under the skin in your upper buttock or abdomen.
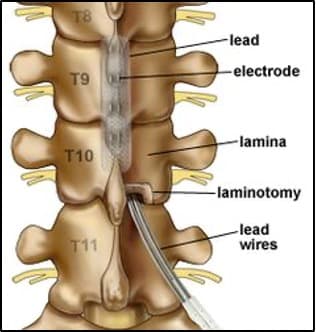
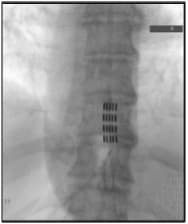
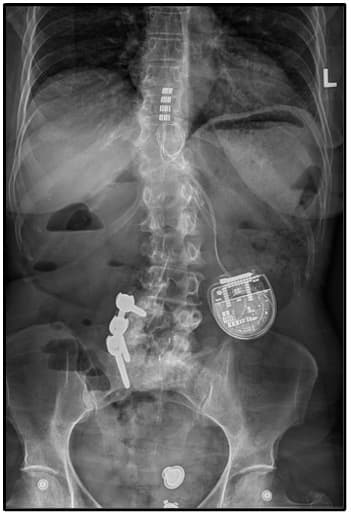
Figure 1: Illustration of the thoracic spine displaying bone removal (inferior laminectomy) and the route of the paddle electrode and wires. X-ray depicting the positioning of the paddle electrode after its placement in the thoracic spine. Another X-ray demonstrating the complete spinal cord stimulator system, featuring the paddle lead within the thoracic spine, connected to a generator positioned above the iliac crest (hip).
Potential Risks and Complications
Risks include, but are not limited to: transient paraplegia, permanent paralysis, bowel and bladder dysfunction, incontinence of bowel and bladder, dural tear with cerebrospinal fluid leak, pseudo-meningocele, post-dural headache, long-term risk of thoracic spine instability, failure to alleviate symptoms, exacerbation of symptoms, inability to replicate trial pain relief, unsuccessful lead placement, paddle lead migration (10 – 25%), wire breakage, hardware failure necessitating re-operation, rotation of pulse generator, persistent pain and numbness, uncomfortable tingling, uncomfortable numbness, chronic pain at generator-battery site, epidural hematoma, subcutaneous hematoma, seroma, and infection. Moreover, risks related to anesthesia, cardiac and pulmonary complications, deep vein thrombosis, and death are present. Additionally, the ability to undergo magnetic resonance imaging (MRI) studies in the future may be compromised.
Note: Studies have demonstrated that the previously mentioned surgical risks escalate if you possess the following factors: obesity (body mass index (BMI) > 30, particularly if BMI > 40), diabetes, a history of prior cardiac issues, usage of blood thinners (for cardiac, stroke, or DVT), and/or multiple concurrent medical conditions.
Recovery and Aftercare
Outpatient surgery: The majority of individuals are discharged on the same day of surgery.
Pain and discomfort: Some soreness or discomfort at the implantation sites is typical and usually lasts about 2 weeks.
Healing time: Complete healing of the incisions might take a few weeks.
Restrictions: Avoid heavy lifting, bending, and twisting for 2 weeks.
Activity: Begin daily walking immediately after surgery. Physical therapy will commence 4 weeks post-surgery, based on patient preference.
Follow-Up Appointments
Follow-up appointments with your healthcare team are usually 2 weeks, 2 months, and annually after surgery to ensure the SCS is working effectively and to address any concerns or adjustments needed.
Long-Term Management
Battery replacement: The generator's battery will require replacement after several years.
Lifestyle considerations: If you intend to undergo MRI scans, notify your doctor, as certain conditions may necessitate special precautions.
Future surgeries: For future surgeries, you must activate the "surgery mode" in your device to prevent monopolar cautery from causing a short circuit in the battery-generator unit. In case this happens, a surgical procedure will be needed to replace the battery.
Outcomes
Studies indicate that approximately 60 — 80% of patients experience a pain reduction of over 50%. The FDA reviewed 107,728 medical drug reports from 2016 to 2020, revealing 497 deaths, 77,937 patient injuries, and 29,294 instances of device malfunction (refer to FDA source). Common patient-reported issues include inadequate pain relief (28%), pain (15%), unexpected therapeutic effects (11%), infection (7.5%), and discomfort (5.9%) (see FDA source). Frequent device-related problems encompass charging issues (11%), impedance (high/low/unspecified) (11%), migration (7%), battery problems (6%), and premature battery discharge (4%). Note: These percentages reflect reported event types and not the estimated proportion of affected patients (FDA).
In our experience, from January 2018 to June 2023, out of 209 patients who received permanent SCS implants, 21 (10.0%) necessitated SCS system explantation for diverse reasons: 13 (6.2%) due to inadequate pain relief, 3 (1.4%) due to infection, 3 (1.4%) due to transient weakness, 1 (0.5%) due to abdominal pain, and 1 (0.5%) due to uncomfortable overstimulation.
Considerations
SCS may not work for everyone, and outcomes can vary. Open communication with your healthcare team is essential throughout the process.
References
- VIDEO: SCS Implantation for Health Care Providers: YouTube Channel, “Round Research and Education Foundation”) (https://youtu.be/NS1Fm1kAV7g)
- Federal Drug Administration, “Conduct a Trial Stimulation Period Before Implanting a Spinal Cord Stimulator (SCS) – Letter to Health Care Providers” (https://www.fda.gov/medical-devices/letters-health-care-providers/condu…)
- Chakravarthy K., et al., Burst Spinal Cord Stimulation: Review of Preclinical Studies and Comments on Clinical Outcomes. Neuromodulation. 2018 Feb 12.
- Ragel, B.T., Riedman, T., McGehee, M, Raslan, A.M., Analysis of Spinal Canal Diameter in the Placement of Thoracic Spinal Cord Stimulator (SCS) Paddle Leads. Pain Practice, accepted June 2023.